ASPEN - Zanubrutinib vs Ibrutinib
Learn more about the ASPEN study and Waldenström’s macroglobulinemia.
The ASPEN trial: zanubrutinib vs ibrutinib for Waldenström’s macroglobulinemia
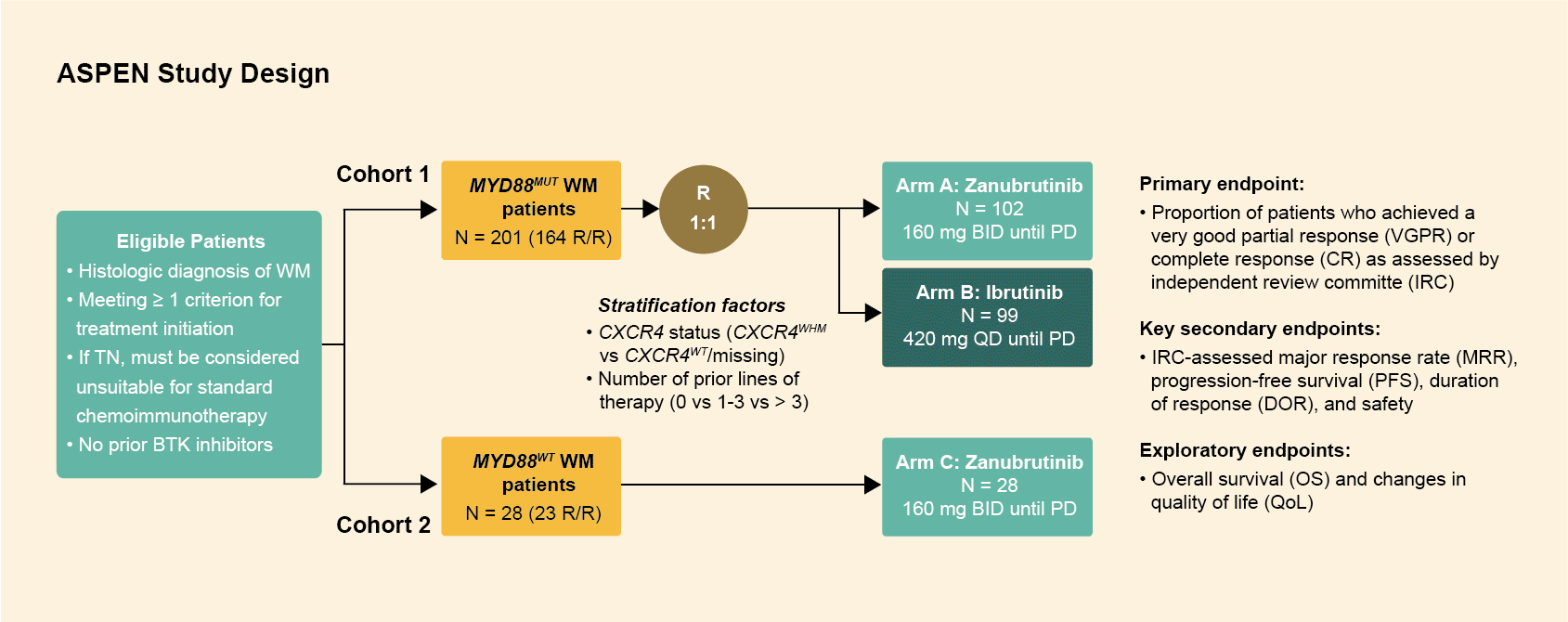
Study design
The ASPEN trial randomized patients with Waldenström's macroglobulinemia to either ibrutinib or zanubrutinib, and included 229 patients (both treatment‑naïve and relapsed or refractory) in 2 cohorts: a randomized cohort (cohort 1) consisting of 201 patients with a MYD88 mutation1 and a non‑randomized cohort (cohort 2) with 28 wild type/undetermined patients.2
- Patients with MYD88L265P disease were assigned 1:1 to receive ibrutinib (420 mg once daily) or zanubrutinib (160 mg twice daily), in 28-day cycles until progressive disease (PD) or intolerance (Cohort 1, N=201).1
- The majority of patients in both arms were relapsed/refractory (R/R; >80%), with a treatment-naïve (TN) minority (<20%).
- Stratification factors included CXCR4 status (CXCR4WHIM/CXCR4WT) and number of lines of previous therapy (0, 1-3, or >3).
- Patients with wild-type MYD88 (MYD88WT) disease/undetermined MYD88 mutation status received zanubrutinib until PD on a third non-randomized arm (Cohort 2, N=28).2
Results – cohort 1 (MYD88L265P)
Responses
- No patient achieved a complete response (CR).
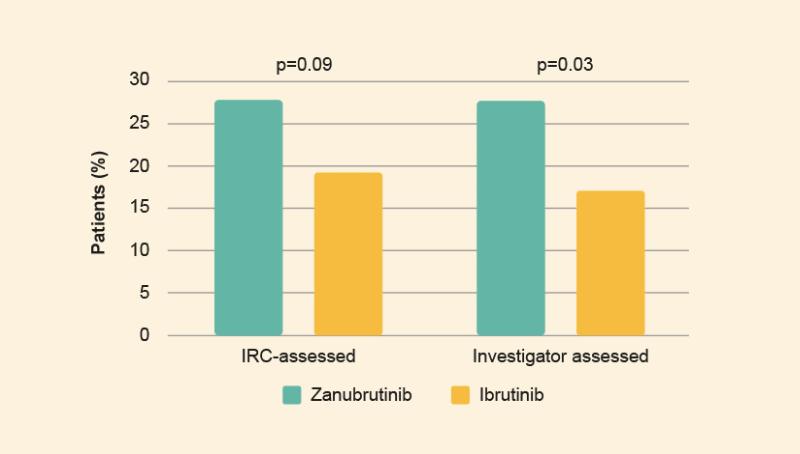
- After a median follow-up time of 19.4 months the frequency of very good partial responses (VGPRs) was higher among zanubrutinib patients than ibrutinib patients (Figure 2).1
Progression-free survival (PFS) and overall survival (OS)
- After median follow-up for PFS of 18.0 and 18.5 months, 15 (15%) zanubrutinib patients and 16 (16%) ibrutinib patients progressed or died.
- Median PFS was not reached for either arm
- Event-free rates at 18 months were comparable: 85% and 84% overall (86% and 82% for R/R patients).
- Six (3 R/R; 3 TN) zanubrutinib patients and eight (8 R/R; 0 TN) ibrutinib patients died; estimated OS rates at 18 months were 97% and 93%, respectively.1
Safety
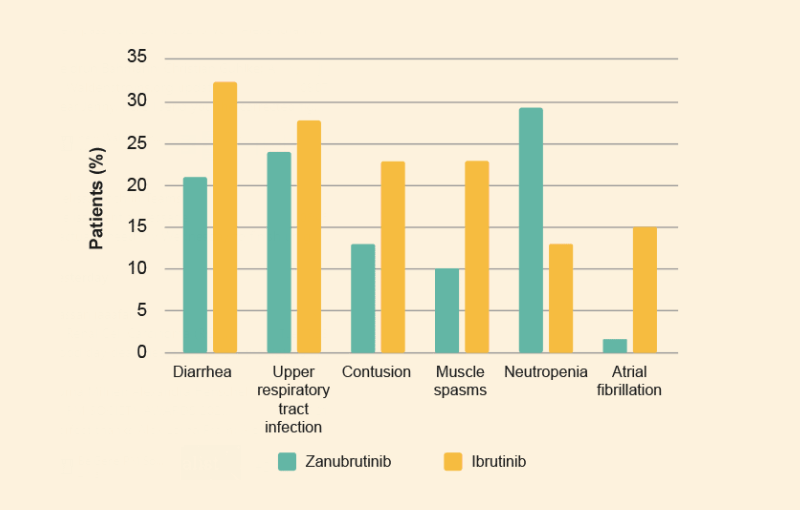
- The most common treatment-emergent adverse events (TEAEs) (reported in >20% of patients) among zanubrutinib patients were neutropenia, upper respiratory infection, and diarrhea (figure 3)
- The most common TEAEs among ibrutinib patients were diarrhea, upper respiratory infection, contusion, and muscle spasms (figure 3).
- Grade ≥3 AEs were reported in 63% and 58% of ibrutinib and zanubrutinib patients, respectively.
- Grade ≥3 hypertension and pneumonia were reported at a ≥5% higher incidence among ibrutinib patients vs zanubrutinib patients.
- Grade ≥3 neutropenia was reported at a ≥5% higher incidence among zanubrutinib patients.1
Results - cohort 2 (MYD88WT)
Responses
- In 26 confirmed MYD88WT patients, the overall response rate (ORR) was 81%, with a major response rate (MRR) of 50.0%, including a VGPR of 27%.2
Progression-free survival (PFS) and overall survival (OS)
- At 18 months, the estimated PFS and OS rates were 68% and 88%, respectively while the median duration of response (DOR) was not reached.2
Safety
- The most frequently reported AEs were diarrhea, anemia, contusion, pyrexia, and upper respiratory tract infection.
- Only two patients discontinued zanubrutinib due to AEs.2
Summary
- Although not statistically significant, a higher rate of IRC-assessed VGPR was observed for zanubrutinib vs ibrutinib (28% vs 19%, respectively) after a median follow-up of 19.4 months in patients with MYD88L265P WM.1
- The incidence and severity of most BTK-associated toxicities (including atrial fibrillation) were lower with zanubrutinib than ibrutinib.
- Zanubrutinib showed clinically meaningful antitumor activity and was considered well tolerated with a low discontinuation rate due to AEs, in patients with MYD88WT WM.2