iNNOVATE – Ibrutinib
Learn more about the iNNOVATE study and Waldenström’s macroglobulinemia.
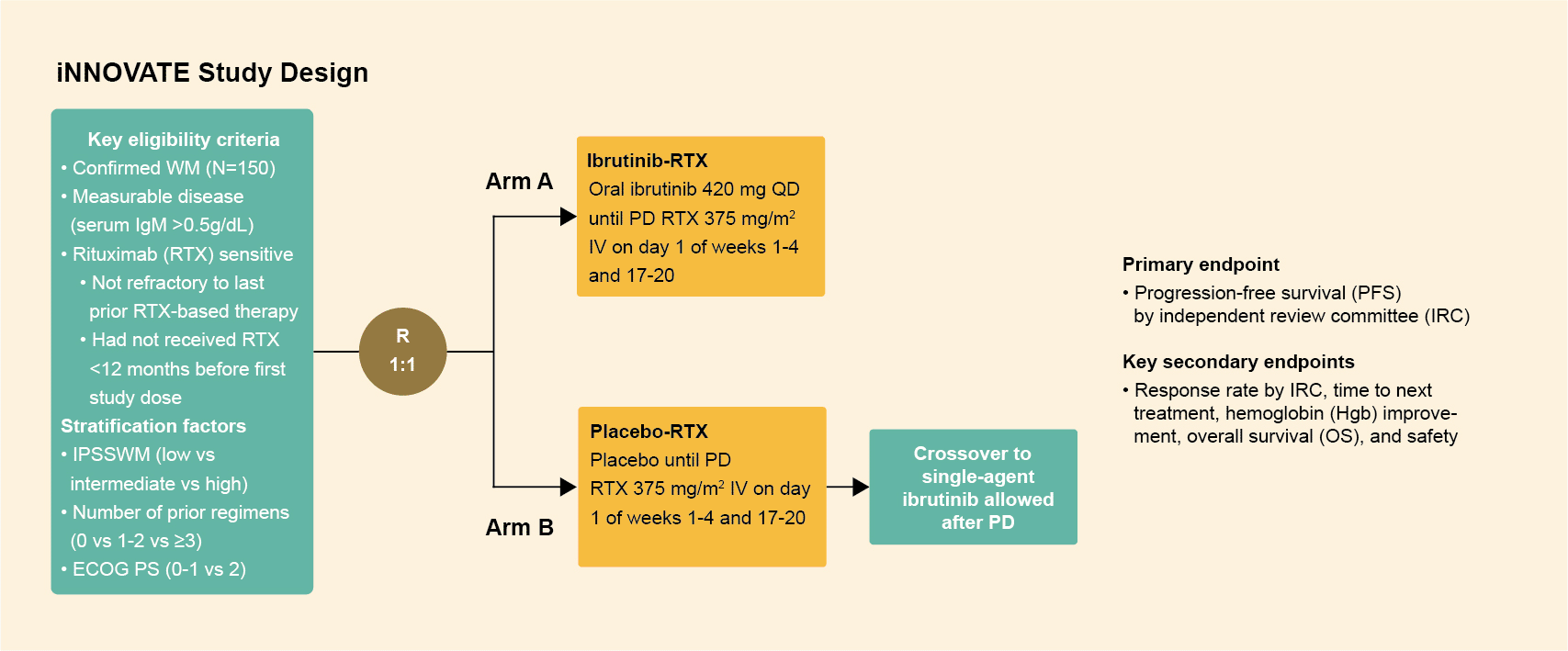
Study design
- The iNNOVATE trial randomized 150 patients with Waldenström's macroglobulinemia (both treatment-naïve patients and those experiencing disease recurrence) to ibrutinib in combination with rituximab or rituximab alone.
- The patients were randomly assigned in a 1:1 ratio to receive either oral ibrutinib (420 mg once daily) or placebo until disease progression or unacceptable toxic effects.
- The two groups also received extended intravenous rituximab (375 mg per square meter of body-surface area, with infusions at weeks 1 to 4 and 17 to 20).1,2
Results
Progression-free survival (PFS) and overall survival (OS)
- The final analysis of iNNOVATE occurred after a median follow-up of 50 months. The results demonstrated a 68% progression-free survival (PFS) in the combination group vs 25% in the rituximab-placebo group at the 54-month time point.
- The median PFS was not reached with ibrutinib-rituximab vs 20.3 months with placebo–rituximab.
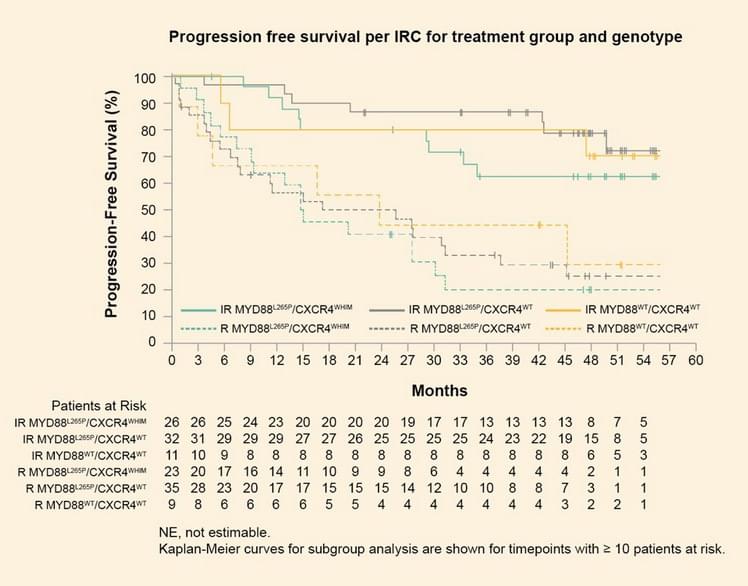
- Patients treated with ibrutinib-rituximab had a PFS benefit that was independent of genotype (HR (95% CI): MYD88L265P/CXCR4WT, 0.18 (0.08–0.43); MYD88L265P/CXCR4WHIM, 0.27 (0.12–0.62); MYD88WT/CXCR4WT, 0.29 (0.07–1.19)) (Figure 2).
- The 54-month overall survival rate was 86% with ibrutinib–rituximab and 84% with placebo–rituximab.
Responses
- The major response rate at 60 months was 76% in the ibrutinib-rituximab arm vs 31% for placebo-rituximab (p<0.0001) and the overall response rate 92% vs 44% (p<0.0001).
- High response rates were observed with combination therapy across genotypes (94% (30/32) in MYD88L265P/CXCR4WT; 100% (26/26) in MYD88L265P/CXCR4WHIM; 82% (9/11) in MYD88WT/CXCR4WT).2
Safety
- Overall, the safety profile was consistent with that of the 30-month follow-up. Minimal differences in adverse effect (AE) rates were observed after 24 months of additional follow-up and AEs even decreased over time.
- The most common grade 3–4 AEs were atrial fibrillation (16%), hypertension (15%), neutropenia (13%), and anemia (12%).2
Summary
- With up to 5 years of follow-up, ibrutinib-rituximab showed ongoing superiority in patients with WM irrespective of key clinical features including genotype.
- Ibrutinib-rituximab maintained a manageable safety profile, with no new safety signals identified and minimal increases in common AEs after an additional 24 months of follow-up.2