PHASE II – Acalabrutinib
Learn more about the latest clinical trials in Waldenström’s macroglobulinemia.
Phase II study of acalabrutinib for Waldenström’s macroglobulinemia
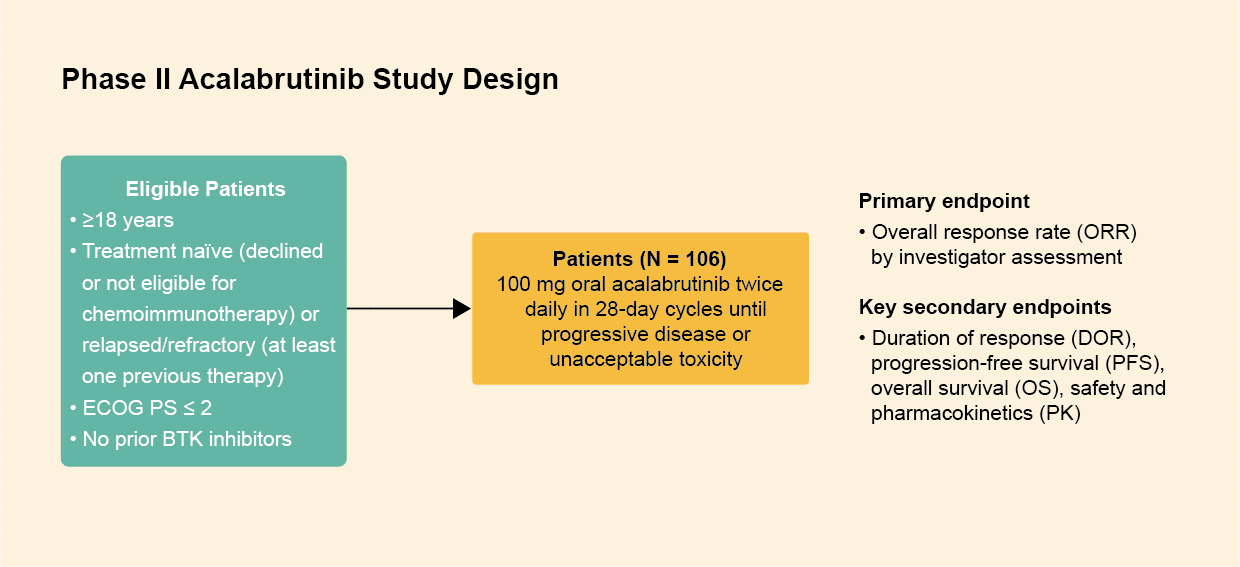
Study design
- In this single‑arm phase II study of acalabrutinib in patients with Waldenström's macroglobulinemia, 106 treatment-naïve or relapsed or refractory patients received 100 mg acalabrutinib twice a day (6 patients received 200 mg once a day but later switched 100 mg twice a day) in 28‑day cycles until progressive disease or intolerance.1
Figure 1: Study design of the phase II acalabrutinib study. Derived from Owen RG, et al. Lancet Haemotology. 2020. 7(2):E112-E121.1
Results
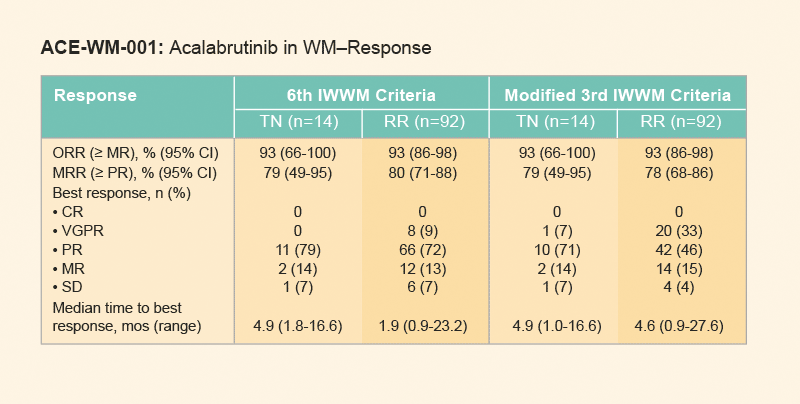
- After a median follow-up of 27·4 months the overall response rate was 93%, with a major response occurring in 78% to 80% of patients
- Progression-free survival (PFS) with acalabrutinib at 24‑month follow-up was 90% in treatment-naïve patients and 82% in the relapsed or refractory group.
- The overall survival was 92% in treatment-naïve patients and 89% in relapsed or refractory patients, in the same time period
Figure 2: Response rates in patients receiving acalabrutinib. Derived from Owen RG, et al. Lancet Haemotology. 2020. 7(2):E112-E121.1
Safety
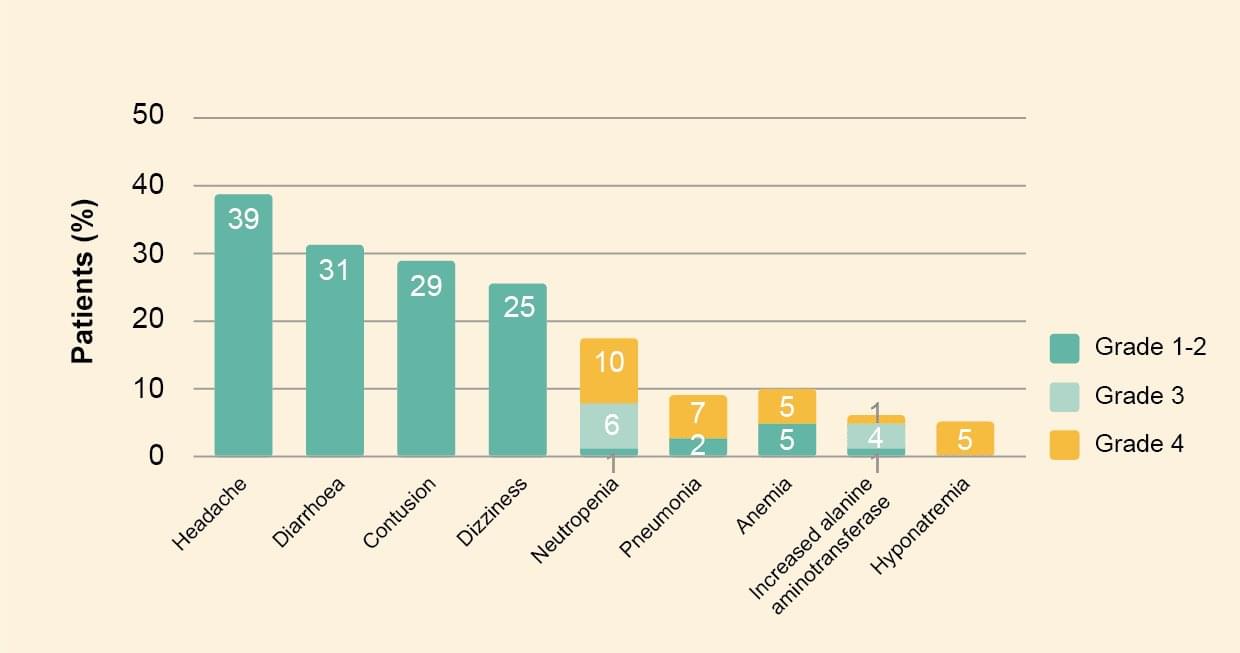
- Common adverse events of any grade were headache, diarrhea, contusion, and dizziness. Common grade 3 and 4 adverse events were neutropenia, pneumonia, anemia, increased alanine aminotransferase, and hyponatremia.
- Of note, bleeding events occurred in 58% of patients and were commonly contusion (29%) and epistaxis (11%).
- Atrial fibrillation occurred in 5 patients and grade 3 hypertension occurred in 3 patients.1
Summary
- This present study demonstrated that acalabrutinib is active as single-agent therapy with a manageable safety profile in patients with treatment-naive, or relapse or refractory Waldenström’s macroglobulinemia.
- Further studies are needed to establish its efficacy against current standard treatments and to investigate whether outcomes can be improved with combination therapies.