Phase II – Tirabrutinib
Learn more about the latest clinical trials in Waldenström’s Macroglobulinemia.
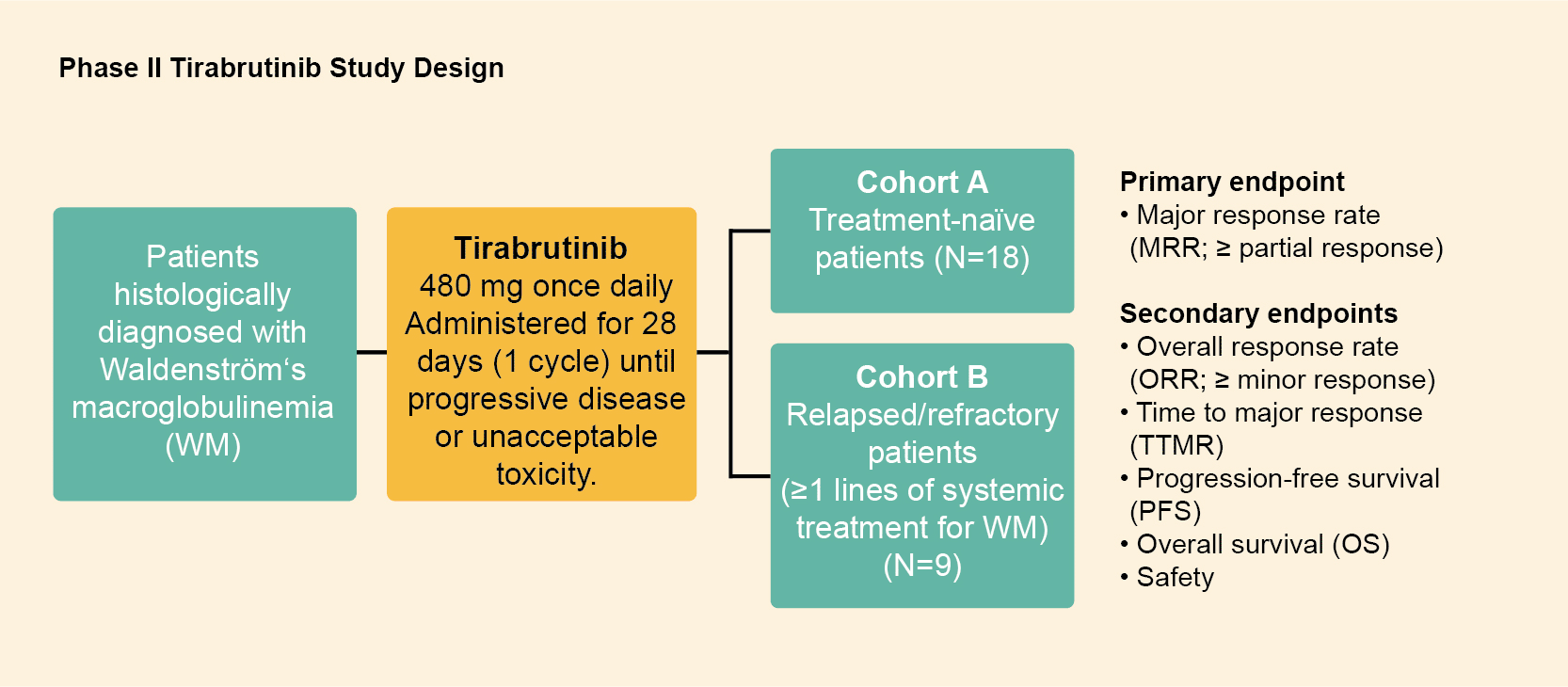
Phase II study of tirabrutinib for Waldenström’s macroglobulinemia
Study design
- The trial was conducted with an open‐label and single‐arm design at 19 sites in Japan
- Inclusion criteria in Cohort A
- Presence of symptomatic WM or serum IgM levels of >4000 mg/dL
- Other major eligibility criteria for both cohorts
- Age ≥ 20 years
- Monoclonal gammopathy with serum IgM levels of >500 mg/dL
- An Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1
- Acceptable laboratory test results
- Major exclusion criteria
- Tumor lesions in the central nervous system (CNS) and prior administration of BTK inhibitors
- Patients were treated with tirabrutinib 480 mg once daily orally under fasting conditions for 28 days as 1 cycle
- Tirabrutinib was continued until disease progression or clinically unacceptable toxicity
Figure 1: Study design of the phase II tirabrutinib study. Derived from Sekiguchi N, et al. Cancer Sci. 2020;111(9):3327-3337.1
Patient population
- Median age was 71 years
- Median serum immunoglobulin M (IgM) level was 3600 mg/dL
- The MYD88L265P mutation was present in 96.2% of patients
Results
Responses
- MRR and ORR were 88.9% and 96.3%, respectively (Figure 2)
- Median time to major response was 1.87 months
PFS and OFS
- PFS and OS were not reached with a median follow‐up of 6.5 and 8.3 months for Cohorts A and B, respectively
Safety
- The most common adverse events (AEs) were rash (44.4%), neutropenia (25.9%), and leukopenia (22.2%)
- Most AEs were classified as grade 1 or 2
- Grade ≥ 3 AEs included neutropenia (11.1%), lymphopenia (11.1%), and leukopenia (7.4%)
- No grade 5 AEs were noted
- All bleeding events were grade 1; none were associated with drug‐related atrial fibrillation or hypertension.
Summary
- Although the follow‐up duration was relatively short, the study met the primary endpoint (major response rate).
- The present study demonstrated that tirabrutinib monotherapy is highly effective with rapid responses and is well tolerated in both treatment-naïve patients and those with relapsed / refractory symptomatic WM.
- Some efficacy endpoints (including PFS and OS) could not be evaluated due to the limited observation period; therefore, future studies with a longer follow‐up period are warranted.