Phase II – Venetoclax
Learn more about the latest clinical trials in Waldenström’s macroglobulinemia.
Phase II study of venetoclax for Waldenström’s macroglobulinemia
Study design
- In a multicenter phase 2 clinical trial evaluating venetoclax in patients with Waldenström’s macroglobulinemia, 31 patients were enrolled.
- The median number of prior therapies was 2 (range 1-10), and 16 patients were previously treated with a BTKi. All patients carried the MYD88L265P mutation and 17 also had a CXCR4 mutation.
Figure 1: Study design of the phase II venetoclax study. Derived from Castillo J, et al. Clinical Lymphoma, Myeloma and Leukemia 2019; 19 (10) Supp E39-E40
Results
IgM, bone marrow involvement and hemoglobin levels
- At baseline, median serum IgM was 3,542 mg/dl (range 642-7,970 mg/dl), median bone marrow involvement was 40% (range 4-95%) and median hemoglobin was 10.6 g/dl (range 6.4-13.5 mg/dl).
- At 12 months, serum IgM declined to 1,071 mg/dl (range 89-4,770 mg/dl), bone marrow involvement declined to 3% (range 0-50%) and hemoglobin increased to 13.1 g/dl (range 8.9-14.9 g/dl).1
Responses
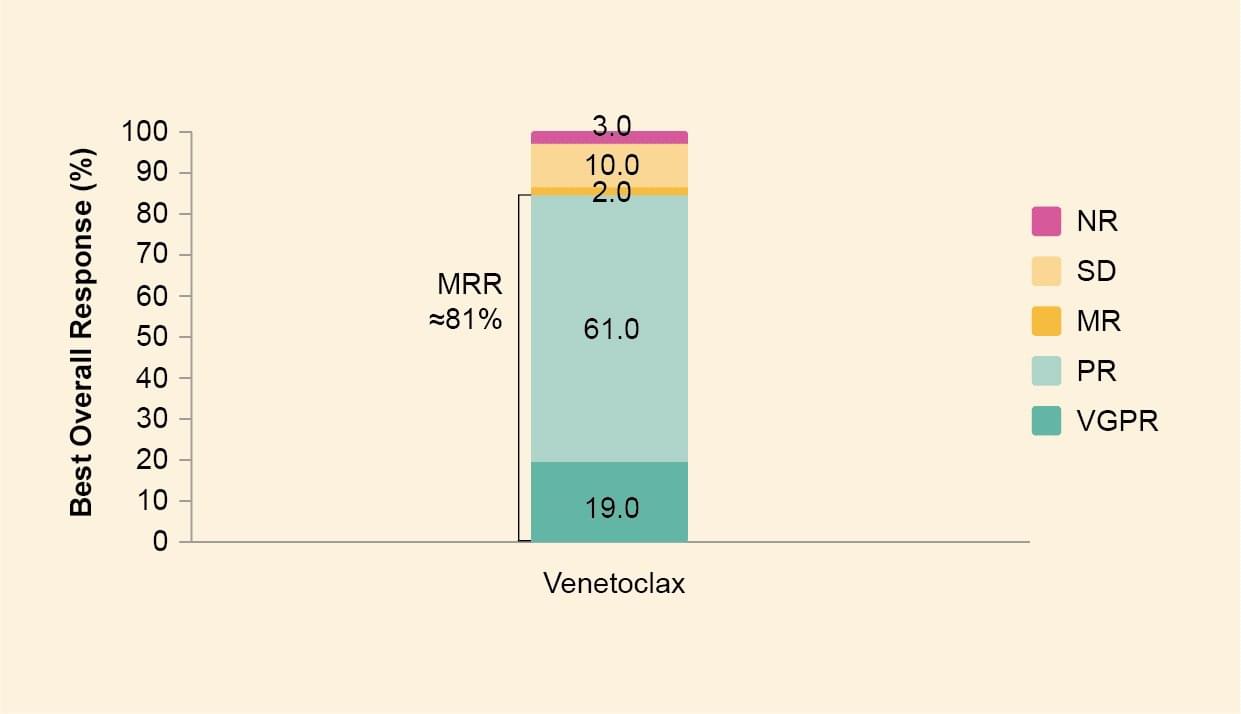
- At best response, very good partial response was attained in 6 patients (19%), partial response in 19 (61%), minor response in 2 (6%), stable disease in 3 (10%) and no response in 1 (3%) (Figure 2).
- The overall response rate was ≈87% and major response rate was ≈81% (rounded values may differ).
- Patients with refractory disease had lower major response rate than patients with relapsed disease (p≤0.005).
- Median time to respond (TTR) was 1.9 months (95% CI 1.1-3.1 months) and was slower in patients with prior BTKi exposure (p<0.001).1
Progression-free survival
- The 2-year PFS rate is 76% (95% CI 52-89%).
- Refractory disease was associated with a worse 2-year PFS (p≤0.003).1
Safety
- Generally, the treatment was well tolerated.
- Grade 4 neutropenia occurred in 5 patients.
- Grade 3 adverse events included neutropenia (n=15), anemia (n=4) and diarrhea (n=4).
- One instance of laboratory TLS occurred.
- Venetoclax was dose reduced due to neutropenia (n=2), fatigue (n=1), diarrhea (n=1) and self-reduction (n=1).
- No IgM flare or clinical TLS were observed, and there have been no deaths.1
Summary
- Venetoclax is a safe and effective treatment option for patients with symptomatic, previously treated WM.
- A study combining ibrutinib and venetoclax in previously untreated patients with Waldenström macroglobulinemia is ongoing (NCT04273139).